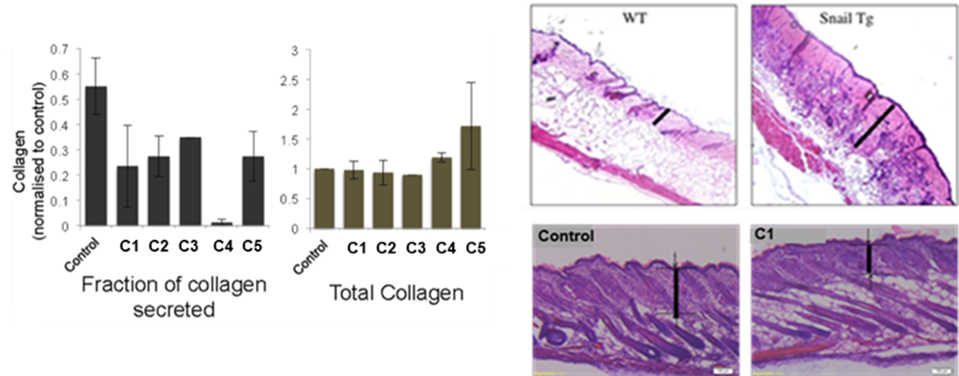
Collagen secretion inhibition in cell and mouse models of fibrosis
Compounds that inhibit the formation of the collagen endoplasmic reticulum (ER)-exit machinery in fibroblasts reducing collagen secretion. They can be used for treating fibrotic diseases by targeting the key, common pathological issue, collagen secretion.
Technology:
Fibrosis underpins the morbidity and mortality of, many serious (chronic) diseases of pivotal organs and is estimated to contribute to 45% of deaths worldwide. This is caused by excessive collagen secretion which can buildup fibrotic tissue, a serious condition where excess connective tissue is formed to the extent that it compromises the function of tissues and sometimes entire organs. There is no cure to reverse fibrosis, and current therapies are insufficiently effective to reduce/halt the chronic progress of fibrosis in these diseases and are mainly only relieving symptoms.
Here researchers at the CRG, in collaboration with the University of Cologne, have shown an entirely unique approach: to block the floodgates of collagen at the cellular level. They have identified a strategy, and developed and validated compounds, against the TANGO1 family of proteins, master regulators of the collagen endoplasmic reticulum-exit machinery that inhibit collagen secretion in various in vitro and in vivo scleroderma models. This could provide a therapeutic approach to fight fibrosis by reducing collagen secretion. Currently, the project is focused towards identifying more hit compounds through high-throughput small molecule screening for hit-to-lead optimization. Compounds and specific regions of the TANGO1 family of proteins targeted by those compounds are protected by a patent application.
Advantages:
- Expandable to the full spectrum of fibrotic diseases. Other targets modulating collagen secretion are either not specific for collagen secretion and/or are limited to one specific collagen type.
- Acts directly at the common pathological hallmark of the disease rather than targeting profibrotic and fibrogenic factors. The highly variable profibrotic and fibrogenic factor-profile and their complex interaction between the broad spectrum of fibrotic diseases, impedes the development of a straightforward and effective therapeutic approach targeting such factors.
References:
Raote, I., Rosendahl, AH., Häkkinen, HM. et al. TANGO1 inhibitors reduce collagen secretion and limit tissue scarring. Nat Commun 15, 3302 (2024). https://doi.org/10.1038/s41467-024-47004-1.


